Milestones in building NanoGroup value
| Project family | Milestone | Estimated date | Okno partneringowe |
| NanOX Recovery Box | Start of certification process | 2026 01 | Q4 2026 – Q1 2027 |
| Obtaining CE certification* | 2026 12 | ||
| NanOX 4 Kidney | Development of the final fluid variant for non‑GLP organ studies | 2026 04 | |
| Selection of a contract manufacturer | 2026 03 | ||
| Selection of contractor for non‑GLP studies – isolated organs | 2026 04 | ||
| Selection of contractor for non‑GLP studies – autotransplantation | 2026 04 | ||
| Manufacturing of the first non‑GMP batch | 2026 07 | ||
| Manufacturing of the first GMP batch | 2026 09 | ||
| Completion of non‑GLP studies for the fluid – isolated organs | 2026 10 | ||
| Completion of non‑GLP studies for the fluid – autotransplantation | 2026 11 | ||
| Dextran conjugates | Completion of in‑vitro studies and formulation selection | 2026 01 | TBA |
| Completion of MTD studies in mice | 2026 03 | ||
| Aptamers | Start of work on an aptamer‑based bioadsorber | 2026 01 | TBA |
| Investment in Auxilius Pharma: AUX-001 | Completion of Phase 1b studies | 2026 Q4 | 2026 Q4 |
Milestones in building NanoGroup value
| Project family | Milestone | Estimated date | Partnering window |
| NanOX Recovery Box | Start of certification process | 2026 01 | Q4 2026 – Q1 2027 |
| Obtaining CE certification* | 2026 12 | Q4 2026 – Q1 2027 | |
| NanOX 4 Kidney | Development of the final fluid variant for non‑GLP organ studies | 2026 04 | Q4 2026 – Q1 2027 |
| Selection of a contract manufacturer | 2026 03 | Q4 2026 – Q1 2027 | |
| Selection of contractor for non‑GLP studies – isolated organs | 2027 04 | Q4 2026 – Q1 2027 | |
| Selection of contractor for non‑GLP studies – autotransplantation | 2027 04 | Q4 2026 – Q1 2027 | |
| Manufacturing of the first non‑GMP batch | 2027 07 | Q4 2026 – Q1 2027 | |
| Manufacturing of the first GMP batch | 2027 09 | Q4 2026 – Q1 2027 | |
| Completion of non‑GLP studies for the fluid – isolated organs | 2027 1o | Q4 2026 – Q1 2027 | |
| Completion of non‑GLP studies for the fluid – autotransplantation | 2027 11 | Q4 2026 – Q1 2027 | |
| Dextran conjugates | Completion of in‑vitro studies and formulation selection | 2026 01 | TBA |
| Completion of MTD studies in mice | 2026 03 | TBA | |
| Aptamers | Start of work on an aptamer‑based bioadsorber | 2026 01 | TBA |
| Investment in Auxilius Pharma: AUX-001 | Completion of Phase 1b studies | 2026 Q4 | 2026 Q4 |
Our leading projects
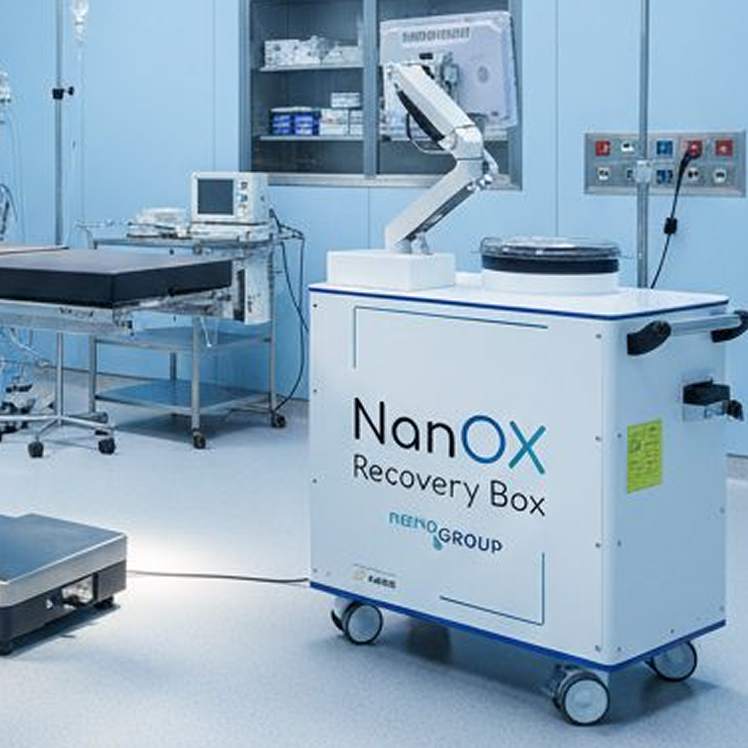
NanOX Recovery Box
The first dedicated normothermic kidney perfusion device on the market. The future gold standard.

NanOX 4 Kidney
A synthetic perfusion fluid dedicated to ex-vivo organ preservation. The first-in-class solution that maintains the key respiratory function of blood. A breakthrough in transplantation.

AUX-001
An improved form of Nicorandil for the treatment of chronic angina pectoris. Simplified FDA 505(b)(2) regulatory pathway, with priority target of the U.S. market.
Pipeline
Key pillars of NanoGroup’s development focused on critical, unmet medical needs.
Project family
Project
Status
Concept
Discovery
Pre-clinical
Respiratory Gas Transport System
NanOX 4 Kidney
- Concept
- Discovery
- Pre-clinical
NanOX Recovery Box
- Concept
- Discovery
- Pre-clinical
NanOX 4 Liver
- Concept
- Discovery
- Pre-clinical
Blood Substitute – Humans
- Concept
- Discovery
- Pre-clinical
Blood Substitute -Veterinary
- Concept
- Discovery
- Pre-clinical
Polysaccharide Nanoparticle Platform
PolEpi
- Concept
- Discovery
- Pre-clinical
Projects Based on Polysaccharide Nanoparticles
- Concept
- Discovery
- Pre-clinical
Conjugates of dextran nanoparticles
TBA
- Concept
- Discovery
- Pre-clinical
Radiotherapeutics
TBA
- Concept
- Discovery
- Pre-clinical
Subsidiaries
dotoMedical and aptamer platform
A patented method for aptamer manufacturing and its applications.
Career
Do you set ambitious scientific goals for yourself? Are you interested in participating in medical projects in the fields of biotechnology and nanotechnology?
NanoGroup is a platform for expertise and specialists united by the belief that breakthrough technologies addressing pressing global challenges can be created and developed in Poland.
If you’d like to join our team, get in touch with us!
Contact us
NanoGroup S.A.
ul. Rakowiecka 36
02-532 Warszawa
NIP: 5213757847
REGON: 365989838
KRS: 0000649960
District Court for the Capital City of Warsaw, XIII Commercial Division of the National Court Register, KRS number 0000649960. Share capital (fully paid) 32 813 238,00 PLN
Office
+48 604 741 303