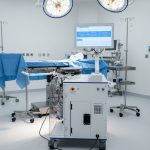
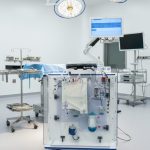
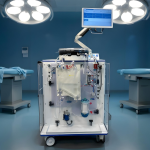
NanoGroup unveils the first unit of the NanOX Recovery Box organ perfusion device. Innovative solution enters engineering testing stage and prepares for certification
NanoGroup S.A., a WSE-listed company developing innovative medical technologies, has unveiled the first unit of the NanOX Recovery Box organ perfusion device, made to medical device compliance standards, as announced. This is a key milestone in the development of the project, paving the way for the start of engineering studies and then the certification process. The procedure at the Notified Body is expected to begin in January 2026, and the market debut of the device, which allows for an increase in the number of organs available for transplantation, is planned for later that year.
The NanOX Recovery Box is an innovative device for extracorporeal kidney perfusion, enabling long-term storage and continuous monitoring. Thanks to its technological flexibility, it can be used for commonly used hypothermic perfusion (e.g., using Belzer fluid), but especially for novel normothermic perfusion (e.g., using red blood cell concentrate). This design makes the device not only versatile and ready to work with currently available perfusants on the market, but also in line with the megatrends of modern transplantology.
– As announced and in line with our R&D schedule, we have completed the construction phase and received the first copy of the NanOX Recovery Box in medical device standard. We are now focusing on engineering research and preparing for the certification process so that the device can hit the market as soon as possible. In parallel, we are in talks with partners interested in distributing it, which significantly increases the potential for scaling sales. We plan that once certified, the NanOX Recovery Box will be commercially available, and we hope this will be as early as next year. – says Piotr Mierzejewski, Vice President of the Management Board of NanoGroup S.A.
An account of the presentation of the first NanOX Recovery Box (September 2025):
Work schedule:
– by the end of 2025: conduct and complete engineering studies at an accredited body and complete technical documentation,
– December 2025: ready clinical evaluation (Clinical Evaluation Report) with results of biocompatibility testing of the perfusion set,
– January 2026: formal start of the certification process at the Notified Body.
NanOX Recovery Box as part of a larger system
NanOX Recovery Box is being developed as part of a broader system, NanOX, which aims to increase the number of organs available for transplantation. The system also includes the NanOX 4 Kidney synthetic perfusion fluid. Although the device itself is an independent project and will be offered separately, its full potential is revealed when combined with the proprietary perfusate. In such a configuration, the NanOX Recovery Box not only enables organ regeneration under near-physiological conditions, but also allows blood to be completely eliminated from the transplantation process, which could realistically change the standards of transplantation.
NanOX 4 Kidney is a unique fluid designed as a functional blood substitute. It contains nanorespirocytes – innovative carriers of respiratory gases, many times smaller than erythrocytes and more efficient in transporting oxygen and carbon dioxide. Unlike blood, the perfusate does not contain leukocytes (involved in the development of inflammatory processes) or pathogens. It is also much easier to store and distribute, which is crucial for transplant logistics.
Certification of NanOX 4 Kidney is planned for 2029. Until then, NanOX Recovery Box will function independently, working with commercially available perfusion fluids.
– NanOX Recovery Box is our first market-ready solution. Its versatility gives it great commercial potential, and when combined with the NanOX 4 Kidney fluid we are developing, it could change the standards of transplantation around the world, saving tens of thousands of lives each year. – emphasizes Piotr Mierzejewski, vice president of NanoGroup S.A.
The work on the NanOX Recovery Box project is financed from its own funds, which ensures stable financing for its further development.
Market outlook
The value of the normothermic perfusion market is confirmed by recent industry deals – an example is the acquisition of UK-based OrganOx by Japan’s Terumo Corporation for $1.5 billion, equivalent to more than 20 times its annual revenue. Against this backdrop, NanoGroup’s solutions stand out with its patent-pending perfusion device and innovative perfusate, featuring a higher level of safety and simplified logistics, making them attractive to both health systems and potential business partners interested in global deployment of the technology.